The ground state electron configuration of chlorine is shown.Which statement concerning an atom of chlorine is FALSE? 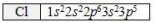
A) A chlorine atom has 17 total electrons.
B) The outermost energy level in a chlorine atom is n=3.
C) A chlorine atom has 5 valence electrons.
D) A chlorine atom needs one electron to obtain an octet in its outermost energy level.
E) A chlorine atom has 17 protons.
Correct Answer:
Verified
Q3: Which period contains the element sodium?
A)one
B)two
C)three
D)five
E)eleven
Q4: Which statement concerning atoms is FALSE?
A)The atomic
Q12: When a neutral atom gains one or
Q16: What is the value of the mass
Q17: The modern periodic law states that the
Q23: Atoms with the biggest radii occur in
Q24: Hydrogen can form two different ions: a
Q26: Which best explains why an Al3+ ion
Q39: What are valence electrons?
A)the electrons located in
Q47: In a neutral atom,what number equals the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents