Which ion is NOT isoelectronic (i.e.has the same electron configuration) with Ar? The electron configuration of Ar is shown. 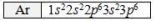
A) Cl−
B) K+
C) Br−
D) Ca2+
E) S2−
Correct Answer:
Verified
Q27: The element carbon forms the basis of
Q30: What is the general name given to
Q31: The Aufbau Principle specifies which of the
Q32: Which statement concerning the elements fluorine,chlorine,bromine,and iodine
Q36: How many valence electrons are present in
Q40: Which statement correctly describes the basis for
Q43: What is a horizontal row of elements
Q45: What Group IA (1)ion has the electronic
Q48: The modern periodic table is arranged according
Q61: Which of the following elements is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents