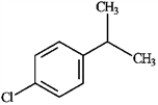
For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband hydrogen-decoupled 13C NMR spectra. Enter the numerical value in the blank provided to the left of the structure.
-_______ 
Correct Answer:
Verified
Q60: How many signals appear in the proton-decoupled
Q61: All of the following compound produce
Q62: Predict the splitting of each of the
Q63: Which C8H10 compound gives the following 1H
Q64: Which C8H10 compound gives the following 1H
Q66: Nuclear magnetic resonance spectroscopy provides information about
Q67: For each of the compounds below tell
Q68: Consider the compound shown below. 
Q69: For each of the compounds below tell
Q70: For each of the compounds below tell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents