Identify the type of coupling that exists between the two protons in the following compound, given that the compound is asymmetrical. 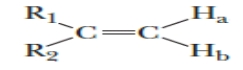
A) Geminal coupling
B) Vicinal coupling
C) Long-range coupling
D) Single-bond coupling
Correct Answer:
Verified
Q89: Identify the physical factor that influences the
Q90: What does "Y" denote in the coupling
Q91: On which factor is chemical shielding not
Q92: Identify the methyl hydrogen with the
Q93: Identify the compound (C3H7Cl) that gives the
Q94: Identify the compound (C8H10) that gives the
Q96: Identify the most appropriate intensity maintained for
Q97: Consider the following structure. Answer the following
Q98: Identify the compound (C8H10) that gives the
Q99: Identify the compound (C4H8O2) that gives the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents