Sample #1 is made from an isotope with decay constant 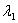 and sample #2 is made from an isotope with decay constant
and sample #2 is made from an isotope with decay constant 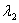 , where
, where 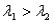 . Which of the following statements must be true?
. Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:
Verified
Q3: Gamma radiation is in fact which of
Q4: Neglecting recoil of the gold nucleus, how
Q6: The relationship Q7: The atomic number of a given element Q8: For every stable nucleus except hydrogen, if Q9: The experiment, which gave the first evidence Q10: The atomic mass number of a nucleus Q11: The molecular mass for chlorine is not Q17: If there are 146 neutrons in 238U,how Q20: A radioactive material initially is observed to![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents