The gas, 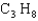 , is used in outside grills, and several moles of this gas will burn during an afternoon's grilling. What is the mass of one molecule of this gas?
, is used in outside grills, and several moles of this gas will burn during an afternoon's grilling. What is the mass of one molecule of this gas?
A) 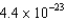 g
g
B) 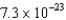 kg
kg
C) 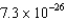 kg
kg
D) 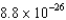 kg
kg
Correct Answer:
Verified
Q2: With volume and molar quantity held constant,by
Q3: An ideal gas is confined to a
Q4: A 4.00-L container holds half a mole
Q7: An ideal gas is confined to a
Q8: In the relationship
Q9: The pressure in a constant-volume gas
Q10: An ideal gas is confined to a
Q13: Four moles of nitrogen gas are contained
Q15: With molar quantity and temperature held constant,by
Q72: For a quantity of ideal gas,which of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents