If the temperature of a gas doubles, by what factor does the speed of a molecule with the average kinetic energy change?
A) It increases by a factor of 2.
B) It increases by a factor of 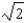 .
.
C) It increases by a factor of 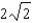 .
.
D) It increases by a factor of 4.
Correct Answer:
Verified
Q47: When a sealed container of ideal gas
Q48: For a monatomic gas, Q49: John rapidly pulls a plunger out of Q50: In a system of ideal gas with Q51: The temperature of a quantity of ideal Q53: What is the internal energy of a Q54: If the average kinetic energy of Q55: For an ideal gas of a given Q56: The internal energy of a monatomic ideal Q57: The noble gases, listed by increasing molecular![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents