What are the bond angles in the structure shown below? 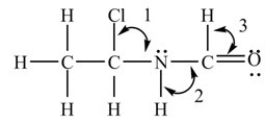
A) Angle 1 = 109.5°,Angle 2 = 109.5°,and Angle 3 = 109.5°
B) Angle 1 = 109.5°,Angle 2 = 120°,and Angle 3 = 120°
C) Angle 1 = 109.5°,Angle 2 = 90°,and Angle 3 = ~109.5°
D) Angle 1 = 109.5°,Angle 2 = ~109.5°,and Angle 3 = 120°
Correct Answer:
Verified
Q49: The functional group of which type of
Q50: How many covalent bonds does nitrogen typically
Q51: What is the correct molecular formula for
Q52: Which representation has the bond polarities properly
Q53: Which compound is most flammable?
A)HOCH2CH2OH
B)NaCl
C)CO2
D)HCl
Q55: Which structure would have the molecular formula
Q56: Which compound has the highest boiling point?
A)HOCH2CH2OH
B)CH3NHCH2CH3
C)CH3CO2CH2CH3
D)NaCH3COO
Q57: Which molecule is a polar molecule?
A)
Q58: Three of the four structures below represent
Q59: Which bonding pattern is NOT typical of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents