The molecule below is an example of an aromatic compound. 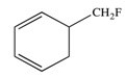
Correct Answer:
Verified
Q56: Which compound has the highest boiling point?
A)HOCH2CH2OH
B)CH3NHCH2CH3
C)CH3CO2CH2CH3
D)NaCH3COO
Q57: Which molecule is a polar molecule?
A)
Q58: Three of the four structures below represent
Q59: Which bonding pattern is NOT typical of
Q60: Based on the functional group present,the compound
Q62: MTBE is soluble in both gasoline and
Q63: Cholesterol is soluble in a nonpolar solvent,such
Q64: The compound represented by the skeletal structure
Q65: The two structures shown below represent the
Q66: The molecule below is an example of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents