Butane,CH3CH2CH2CH3,has the structure shown below. What is the strongest type of intermolecular force that exists between two butane molecules? 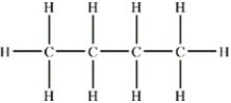
A) London dispersion forces
B) Hydrogen bonding
C) Temporary dipole interactions
D) Dipole-dipole interactions
Correct Answer:
Verified
Q20: An aerosol can has a pressure of
Q21: Which molecule(s)exhibit London dispersion forces?
A)Kl
B)CH4
C)NH3
D)HBr
E)All of the
Q22: Surface tension measures which of the following?
A)A
Q23: Three of the four phase changes below
Q24: Which molecule(s)exhibit hydrogen bonding?
A)H2S
B)CH4
C)NH3
D)HCl
E)All of the molecules
Q26: Which is an ionic solid?
A)KCl
B)Cu
C)SiO2
D)Rubber
E)Polyethylene
Q27: Which is a molecular solid?
A)MgCl2
B)Au
C)Glass
D)Graphite
E)Sucrose (C12H22O11)
Q28: What volume does 7.50 × 1020 molecules
Q29: The normal boiling point of a liquid
Q30: A sample of gas contains four gases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents