Which of the following compounds has the highest boiling point?
A) 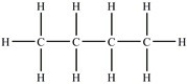
B) 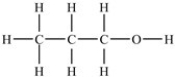
C) 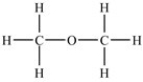
D) 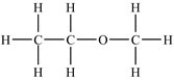
Correct Answer:
Verified
Q30: A sample of gas contains four gases
Q31: Consider the two liquids A and B
Q32: Which phase change below is endothermic?
A)Deposition
B)Melting
C)Condensation
D)Freezing
Q33: Propanol,CH3CH2CH2OH,has the structure shown below. What is
Q34: Which is a network solid?
A)NaBr
B)Ag
C)SiO2
D)Na
E)Sucrose (C12H22O11)
Q36: Which molecule has the lowest surface tension?
A)
Q37: Consider the two liquids A and B
Q38: Which compound has the lowest boiling point?
A)
Q39: Which molecule(s)exhibit hydrogen bonding?
A)CH4
B)CHCl3
C)NF3
D)HF
E)All of the molecules
Q40: What is the volume of 62.3 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents