Consider the two liquids A and B shown in closed containers. Which liquid exhibits the stronger intermolecular forces? 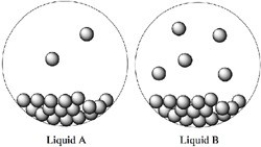
A) Liquid A
B) Liquid B
C) Both Liquids A and B have equal vapor pressures.
D) Not enough information is given.
Correct Answer:
Verified
Q32: Which phase change below is endothermic?
A)Deposition
B)Melting
C)Condensation
D)Freezing
Q33: Propanol,CH3CH2CH2OH,has the structure shown below. What is
Q34: Which is a network solid?
A)NaBr
B)Ag
C)SiO2
D)Na
E)Sucrose (C12H22O11)
Q35: Which of the following compounds has the
Q36: Which molecule has the lowest surface tension?
A)
Q38: Which compound has the lowest boiling point?
A)
Q39: Which molecule(s)exhibit hydrogen bonding?
A)CH4
B)CHCl3
C)NF3
D)HF
E)All of the molecules
Q40: What is the volume of 62.3 g
Q41: Atmospheric pressure in interstellar space is approximately
Q42: If the lungs of a child hold
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents