When octane evaporates,what kind of attractive forces are overcome? 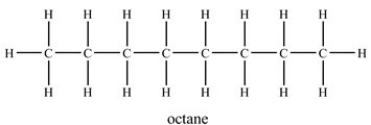
A) Covalent bonding between carbon and hydrogen atoms
B) Hydrogen bonding between octane molecules
C) Dipole-dipole interactions between octane molecules
D) London dispersion forces between octane molecules
Correct Answer:
Verified
Q41: Atmospheric pressure in interstellar space is approximately
Q42: If the lungs of a child hold
Q43: The value of the universal gas constant,R,changes
Q44: When a sample of gas is compressed
Q45: London dispersion forces are the strongest type
Q47: A gas cylinder containing 3.88 mol of
Q48: London dispersion forces are exhibited by all
Q49: The stronger the intermolecular forces,the lower the
Q50: Hydrogen peroxide decomposes to give water and
Q51: At rest,the volume of air in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents