The molecular art depicts the following reversible reaction at equilibrium: CO(g) + Cl2(g)  COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction?
COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction? 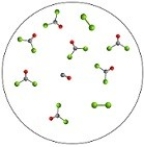
A) K < 1
B) K ~ 1
C) K > 1
D) K = 0
Correct Answer:
Verified
Q35: Which K value below is consistent with
Q36: What is the gas phase chemical reaction
Q37: Which of the following will increase the
Q38: Consider the reaction: PCl3(g)+ Cl2(g) 
Q39: Which K value below is consistent with
Q41: A reversible reaction has reached equilibrium when
Q42: Bond breaking is endothermic.
Q43: Exothermic reactions involve the formation of products
Q44: The difference in energy between the reactants
Q45: The hydrolysis of sucrose depicted below has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents