Which of the following is NOT a reasonable assumption about the chemical reaction whose energy diagram is depicted below? 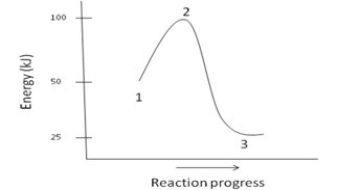
A) The activation energy for the reaction is 100kJ.
B) The reaction is exothermic.
C) ΔH= -25kJ
D) The reaction is favorable.
Correct Answer:
Verified
Q37: Energy is the capacity to do work.
Q52: Which statement concerning the reversible reaction 2
Q53: The energy of the reacting molecules affects
Q54: The stronger the bond,the higher its bond
Q56: The rusting of iron is described by
Q58: One step in the metabolism of glucose
Q59: Which of the following is ALWAYS necessary
Q60: Changes in potential energy occur in chemical
Q61: Consider the following reversible reaction at equilibrium:
Q62: Consider the following reversible reaction at equilibrium:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents