Which statement concerning the reversible reaction 2 NO2(g) 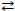 N2O4(g) is true?
N2O4(g) is true?
A) NO2 is the product of the forward reaction.
B) The reverse reaction produces N2O4.
C) At the start of the reaction,the forward and reverse reaction rates are equal.
D) As the forward reaction progresses and more N2O4 is formed,the reverse reaction rate increases.
Correct Answer:
Verified
Q37: Energy is the capacity to do work.
Q47: Breaking a chemical bond requires energy.
Q48: The rate of a chemical reaction increases
Q49: Bond dissociation energies are always positive numbers.
Q50: Ammonia (NH3)is synthesized by the reaction of
Q51: An endothermic reaction is one in which
Q53: The energy of the reacting molecules affects
Q54: The stronger the bond,the higher its bond
Q56: The rusting of iron is described by
Q57: Which of the following is NOT a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents