In the energy diagram shown below,the ΔH of the reaction is labeled by D. 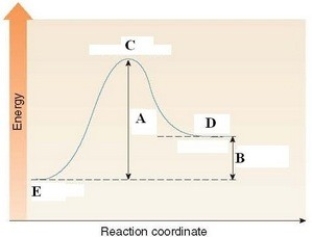
Correct Answer:
Verified
Q60: Changes in potential energy occur in chemical
Q61: Consider the following reversible reaction at equilibrium:
Q62: Consider the following reversible reaction at equilibrium:
Q63: Increasing the concentration of the reactants in
Q64: Consider the following reversible reaction at equilibrium:
Q66: When the equilibrium constant for a reaction
Q67: Increasing the temperature of a reaction mixture
Q68: Chemical reactions are considered favorable if the
Q69: Once equilibrium is reached in a chemical
Q70: A reversible reaction in which K =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents