In the energy diagram shown below,C labels the ________. 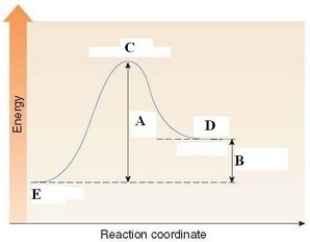
Correct Answer:
Verified
Q82: A reversible reaction is said to have
Q83: Consider the reaction: 2 C2H6(g)+ 7 O2(g)→
Q84: Consider the reaction: 2 C2H6(g)+ 7 O2(g)→
Q85: When reactants can come together and form
Q86: An enzyme contains a region called its
Q88: In order to cause an endothermic equilibrium
Q89: When ΔH is negative,the bonds formed in
Q90: Consider the reversible reaction: CO(g)+ Cl2(g)
Q91: A manufacturing company requires 157 kJ of
Q92: When heat is added to an exothermic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents