A 100-g sample of the compound below contains less than 6.02 × 1023 molecules. 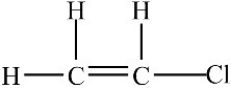
Correct Answer:
Verified
Q80: To determine the overall percent yield in
Q81: Consider the unbalanced chemical equation: NH3 +
Q82: To multiply two numbers in scientific notation,multiply
Q83: In a redox reaction,the _ agent is
Q84: Aluminum metal and oxygen gas are obtained
Q86: In a two-step synthesis where the first
Q87: In the balanced reaction: 2 I- +
Q88: One term in a balanced chemical equation
Q89: A chemical change alters the chemical composition
Q90: A 200-mg ibuprofen (C13H18O2)tablet contains greater than
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents