How many lone pairs of electrons need to be added to the Lewis structure of carbonic acid shown below? 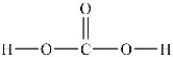
A) 0
B) 3
C) 4
D) 6
E) 7
Correct Answer:
Verified
Q42: Considering the electronegativity values indicated for each
Q43: How many total valence electrons does the
Q44: Phosphorus usually forms two covalent bonds in
Q45: Atoms with three valence electrons generally form
Q46: Which of the statements concerning compounds is
Q48: The covalent bond between chlorine and iodine
Q49: What is the total number of bonding
Q50: Which statement concerning chemical bonds is FALSE?
A)A
Q51: Which atom(s)in the structure below has(have)a partial
Q52: Bonding is the joining of two atoms
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents