The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is INCORRECT? 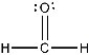
A) Two electrons are being shared between the carbon atom and the oxygen atom.
B) The oxygen atom has four valence electrons that are not being shared with another atom.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have filled valence shells.
Correct Answer:
Verified
Q53: A Lewis structure shows the connectivity between
Q54: Which compound has the greatest number of
Q55: A molecule is a discrete group of
Q56: How many total valence electrons does the
Q57: Atoms with seven valence electrons typically form
Q59: Every atom must have an octet of
Q60: The Lewis structure shown below is not
Q61: The formula for dinitrogen pentoxide is N5O2.
Q62: In the valence shell electron pair repulsion
Q63: The Lewis structure for BH3 contains an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents