What is the chemical formula for the following Lewis structure? 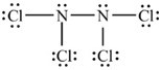
Correct Answer:
Verified
Q82: Carbon tetrachloride has _ valence electrons.
A)4 (four)
B)8
Q83: In the Lewis structure of a molecule,oxygen
Q84: When writing Lewis structures,the symbol below is
Q85: The Lewis structure for the molecule below
Q86: Electronegativity _ down a column of the
Q88: In Lewis structures,fluorine atoms generally do not
Q89: In general,a _ bond will be one
Q90: Dibromomethane (CH2Br2)is a nonpolar molecule.
Q91: Double bonds and triple bonds are never
Q92: A N-O bond is more polar than
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents