The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.According to this figure, what happens when H3O+ is added to the HF/F- buffer? 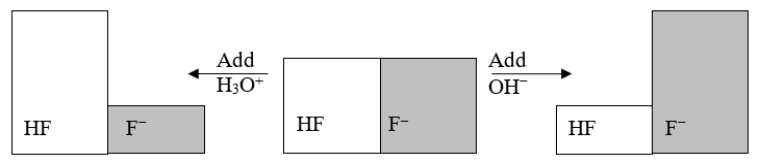
A) The pH decreases.
B) The pH increases.
C) The concentration of HF increases.
D) The concentration of F- increases.
E) Nothing happens.
Correct Answer:
Verified
Q8: Which statement BEST describe a neutralization reaction?
A)
Q9: Which statement does NOT correctly describe pH?
A)
Q10: Generally, strong bases are hydroxide salts of
A)
Q11: Which of the following types of molecules
Q12: A blood sample has a pH of
Q14: What is the concentration of H3O+ and
Q15: Which solution has the highest concentration of
Q16: The boxed species in the following reaction
Q17: The following figure illustrates the action of
Q18: A conjugate acid-base pair is
A) the reactants
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents