The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.Which of the following chemical equations represents the reaction that occurs when OH- is added to the HF/F- buffer? 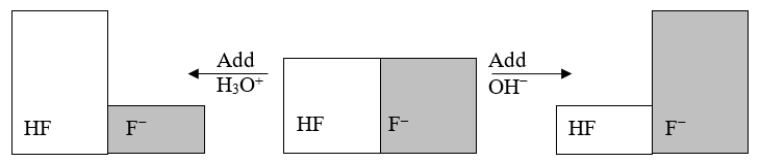
A) HF + H2O ⇌ F- + H3O+
B) F- + H2O ⇌ F- + OH-
C) HF + OH- ⇌ F- + H2O
D) F- + 2 OH- ⇌ HF + O2
E) F- + OH- ⇌ HOF
Correct Answer:
Verified
Q38: Which of the following statements BEST describes
Q39: The reaction between acetic acid and water
Q40: If the concentration of hydronium ion in
Q41: Which species in the following neutralization reaction
Q42: What is the pH of a solution
Q44: Which of the following atomic diagrams best
Q45: Water can react as both an acid
Q46: Consider a buffer solution containing CH3COO-Na+ and
Q47: What happens to pH when the buffer
Q48: The neutralization reaction of potassium hydrogen carbonate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents