Consider solutions A and B separated by a semipermeable membrane.To which volume would pressure need to be applied to prevent osmosis from occurring? 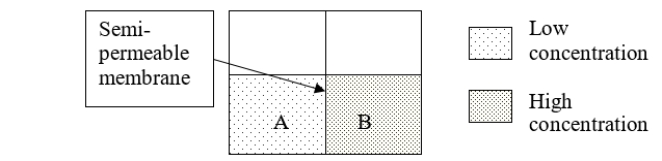
A) A
B) B
C) first A, then B
D) first B, then A
E) It is not possible to prevent this process from occurring.
Correct Answer:
Verified
Q47: In hemodialysis, the solution used in the
Q48: According to a blood test, a patient
Q49: Which statement describes one way in which
Q50: Jane Doe's blood test showed that her
Q51: Capsaicin is responsible for the heat of
Q53: The permissible level of arsenic in drinking
Q54: Which statement explains why whole blood has
Q55: Which figure BEST represents the dissolution of
Q56: A wine label states that it is
Q57: Which of the following does NOT occur
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents