Does isopropyl alcohol have a higher or lower boiling point than acetone? 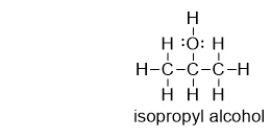
A) higher, because isopropyl alcohol can hydrogen bond
B) lower, because isopropyl alcohol can hydrogen bond
C) higher, because isopropyl alcohol can form dispersion forces
D) lower, because isopropyl alcohol can form dispersion forces
E) It is not possible to determine the answer based on the information provided.
Correct Answer:
Verified
Q32: Are any bonds in acetone, shown below,
Q33: Which of the following is NOT a
Q34: Which tends to be larger for a
Q35: Which of the following physical changes is
Q36: What happens when a large volume of
Q38: A sample of gaseous neon has a
Q39: What is the strongest intermolecular force that
Q40: Which of the following circumstances does NOT
Q41: Which of the following processes is an
Q42: Formaldehyde, commonly used to preserve biological specimens,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents