A gas having a volume of 11.2 L has added to it 0.5 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.Which relationship is used to determine the number of moles of gas originally?
A) 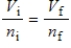
B) 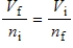
C) 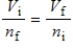
D) 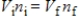
E) There is no relationship between the moles of air and the volume of air.
Correct Answer:
Verified
Q24: What happens to acetone when it is
Q25: Arrow B points to Q26: Water has a higher heat of vaporization Q27: Consider a warm summer's day at the Q28: Which has a higher partial pressure of Q30: The temperature of a gas at 1.00 Q31: What happens to the speed of molecules Q32: Are any bonds in acetone, shown below, Q33: Which of the following is NOT a Q34: Which tends to be larger for a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents