To accomplish the change of phase shown in the diagram below, you could 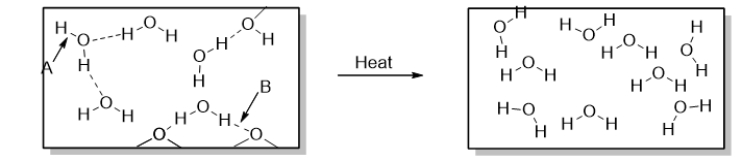
A) drink a glass of water.
B) put water in the freezer.
C) boil water.
D) let an ice cube melt in a warm glass of water.
E) let an ice cube melt in the sink.
Correct Answer:
Verified
Q53: A balloon at STP is heated.Which of
Q54: What change of phase is represented by
Q55: What occurs over the course of the
Q56: What is the volume of 2.01 ×
Q57: How many calories of heat are required
Q59: Which of the following statements BEST describes
Q60: Why is water (H2O)a liquid at room
Q61: A gas having a volume of 11.2
Q62: How do phase changes differ from chemical
Q63: According to Avogadro's law, ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents