According to Avogadro's law, 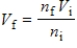 .What is the meaning of this law?
.What is the meaning of this law?
A) Adding moles of gas decreases the volume of the gas.
B) Adding moles of gas increases the volume of the gas.
C) The volume is inversely proportional to the moles of air.
D) Gas particles shrink when they are crowded.
E) Gas particles expand when they are crowded.
Correct Answer:
Verified
Q58: To accomplish the change of phase shown
Q59: Which of the following statements BEST describes
Q60: Why is water (H2O)a liquid at room
Q61: A gas having a volume of 11.2
Q62: How do phase changes differ from chemical
Q64: Because gases are compressible, they become _
Q65: Which statement about vaporization and evaporation is
Q66: Which of the following statements BEST describes
Q67: At the grocery store, a bag of
Q68: In which of the following phase changes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents