The two beakers below each have added to them the same amount of heat energy.Which statement BEST describes what would happen to the temperatures of the two beakers? 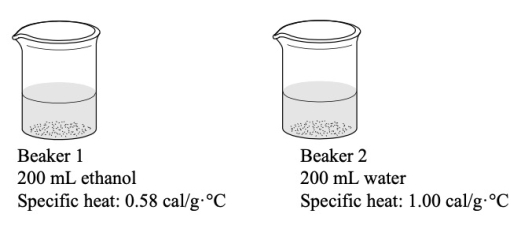
A) The temperature of the two beakers will remain the same.
B) The temperatures of the two beakers will increase by the same amount.
C) The temperature of beaker 1 will increase more than that of beaker 2.
D) The temperature of beaker 2 will increase more than that of beaker 1.
E) It is not possible to predict how the temperature of the beakers will change.
Correct Answer:
Verified
Q78: The relationship between concentration and pressure in
Q79: A scuba diver dives down to 15
Q80: The total pressure in a mixture of
Q81: Which of the following changes of state
Q82: The temperature of a gas at 1.00
Q84: A copper pipe with a mass of
Q85: Balloon A is placed into a container
Q86: Which of the following processes is an
Q87: Which statement BEST describes why a higher
Q88: The atmospheric pressure in Denver is 0.85
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents