How would this diagram be different if the reaction was exothermic? 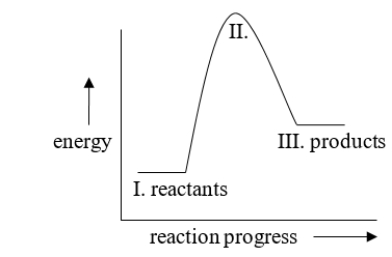
A) III would be lower than I
B) No difference; this reaction is already exothermic.
C) II would be lower than III.
D) II would be lower, between I and III.
E) II would be lower than I.
Correct Answer:
Verified
Q1: Which of the following is a potential
Q2: The energy difference between I and III
Q4: How is a biochemical pathway different than
Q5: The following reaction is a reversible reaction.Which
Q6: A calorimeter is used to measure the
Q7: Combustion reactions are _ because products of
Q8: During anabolism, heat is absorbed.What does the
Q9: Which of the following statements BEST describes
Q10: Each of the following figures represents a
Q11: Which of the following changes increases the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents