The ΔH for the reaction described by the energy diagram below is 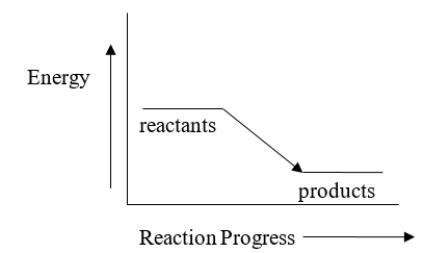
A) very large.
B) greater than zero but not necessarily very large.
C) exactly zero.
D) less than zero.
E) It is not possible to predict anything about the ΔH of this equation.
Correct Answer:
Verified
Q21: An overview of metabolism is shown in
Q22: What is a biochemical pathway?
A) the path
Q23: What type of human calorimetry is the
Q24: What are biological catalysts called?
A) proteins
B) nucleic
Q25: The reaction of water with ammonia is
Q27: A spirometer is used in indirect calorimetry.What
Q28: An endothermic reaction absorbs heat when it
Q29: During anabolism, heat is absorbed.What does the
Q30: The first law of thermodynamics states that
A)
Q31: Which of the following processes are anabolic?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents