Which of the following reactions could be described by the energy diagram below? 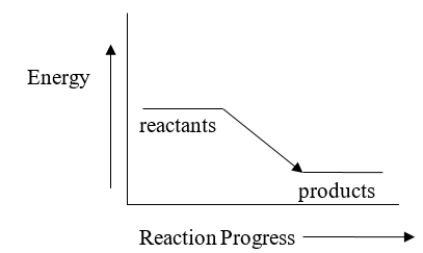
A) 2 CO2 + 556 kJ→ 2 CO + O2
B) CH4 + 2 O2 → CO2 + 2 H2O + heat
C) 8 H2S + heat → 8 H2 + S8
D) 6 CO2 + 6 H2O + heat → C6H12O6 + 6 O2
E) All of the reactions can be described by this energy diagram.
Correct Answer:
Verified
Q28: An endothermic reaction absorbs heat when it
Q29: During anabolism, heat is absorbed.What does the
Q30: The first law of thermodynamics states that
A)
Q31: Which of the following processes are anabolic?
A)
Q32: Which of the following statements describes an
Q34: During catabolism of food, heat is released.Where
Q35: The chemical reaction in the diagram below
Q36: Which of the following molecules is a
Q37: Which of the following diagrams illustrates an
Q38: Which part of the following energy diagram
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents