The energy difference between I and II in the diagram is called 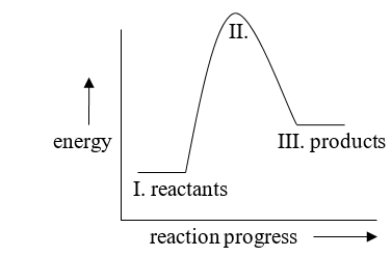
A) the activation energy
B) kinetic energy
C) heat of reaction
D) exothermic energy
E) endothermic energy
Correct Answer:
Verified
Q69: Bond breaking is always endothermic and yet
Q70: The reaction between acetic acid (CH3COOH)and methanol
Q71: Increasing the temperature of an exothermic reaction
Q72: Which statement is the BEST definition of
Q73: Select the TRUE statement concerning a reaction
Q75: How is the reaction that occurs in
Q76: Which bonds are broken, and which bonds
Q77: How many joules of energy are contained
Q78: Increasing the temperature of a reaction causes
Q79: Convert 3.5 × 104 J to calories.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents