Jane Doe has a cholesterol (C27H46O) count of 178 mg/dL.You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood.Which of the following calculations is used to solve this problem?
A) 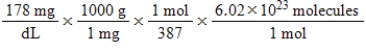
B) 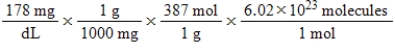
C) 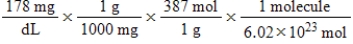
D) 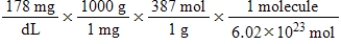
E) 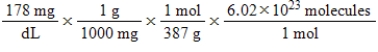
Correct Answer:
Verified
Q7: The balanced equation for the reaction of
Q8: What is the primary relationship between a
Q9: Pentane (C5H12)reacts with oxygen gas (O2)to form
Q10: How many molecules of chlorine must be
Q11: What does it mean for mass to
Q13: If Jane Doe has a blood carbon
Q14: Pentane (C5H12)reacts with oxygen gas (O2)to form
Q15: Under what circumstances is mass conserved?
A) Mass
Q16: What type of reaction occurs when hydrogen
Q17: Calculate the mass of one mole of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents