Which of the following equations is used to convert 91.0 milligrams of urea (CH4N2O) to moles of urea?
A) 
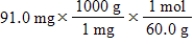
B) 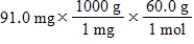
C) 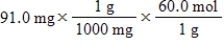
D) 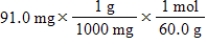
E) 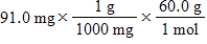
Correct Answer:
Verified
Q61: Which of the following is a consideration
Q62: How many moles of carbon are in
Q63: Which statement BEST describes why we use
Q64: Which statement describes how molecular mass and
Q65: The reaction that occurs in a hydrogen
Q67: What is the mass of one mole
Q68: Which of the following is NOT indicated
Q69: The balanced equation for the combustion of
Q70: How many carbon atoms are in 1
Q71: Is methane (CH4)undergoing oxidation or reduction during
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents