A common, over-the-counter antacid is Al(OH) 3.This antacid reacts with gastric juice (HCl) in the stomach, producing AlCl3 and H2O.Which of the following equations can be used to correctly determine how much gastric juice (HCl) reacts with an antacid tablet containing 0.25 grams Al(OH) 3? Note that you will need to write a balanced chemical equation to answer this question.
A) 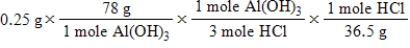
B) 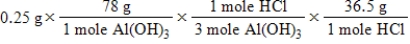
C) 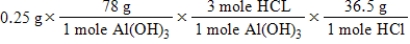
D) 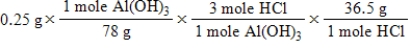
E) 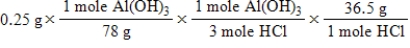
Correct Answer:
Verified
Q78: Hydrogen peroxide loses its potency when it
Q79: The following chemical equation, the decomposition of
Q80: What is the meaning of the arrow
Q81: Which of the following must occur for
Q82: A normal blood oxygen (O2)level is 8.0
Q83: Which of the following values is Avogadro's
Q85: The following chemical equation is not balanced.What
Q86: Which of the following reactions is NOT
Q87: The industrial process for making ammonia from
Q88: How many atoms of oxygen are present
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents