Is the ketone oxidized or reduced during this reaction, and how can you tell? 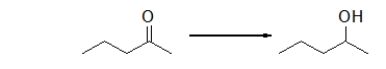
A) Neither.This is not an oxidation-reduction reaction.
B) The ketone is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The ketone is oxidized because it loses two hydrogens.
D) The ketone is reduced because it loses two hydrogens.
E) The ketone is reduced because it gains two hydrogens.
Correct Answer:
Verified
Q40: Fortified foods are
A) foods naturally high in
Q41: What is the product of the following
Q42: What does [H] represent in the following
Q43: Which statement BEST describes a similarity between
Q44: What is the product of the following
Q46: FAD and NAD+ are examples of
A) enzymes.
B)
Q47: What is the product of the following
Q48: The sequence, ethanol → ethanal (acetaldehyde)→ ethanoic
Q49: What type of reaction is this?
Q50: Sorbitol is a sweetener often used in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents