Is the alkene oxidized or reduced during this reaction, and how can you tell? 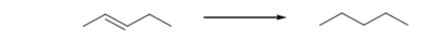
A) Neither.This is not an oxidation-reduction reaction.
B) The alkene is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The alkene is oxidized because it loses two hydrogens.
D) The alkene is reduced because it loses two hydrogens.
E) The alkene is reduced because it gains two hydrogens.
Correct Answer:
Verified
Q72: What is the product of the following
Q73: After an amide is hydrolyzed, it undergoes
Q74: Which amide has a line through the
Q75: Which statement BEST describes the distinction between
Q76: Which classification of alcohols cannot be oxidized,
Q77: What is the product of the following
Q78: Hydrolysis is an example of an acyl
Q79: Which statement describes a difference between FAD/FADH2
Q80: Which molecule can be oxidized to a
Q82: Consumption of ethanol represents "empty calories" because
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents