Do you expect a soap molecule to be more or less soluble than a carboxylic acid with the same number of carbons shown below? 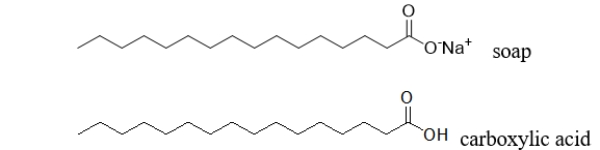
A) less soluble because the soap has a higher molecular weight
B) more soluble because the soap has a higher molecular weight
C) less soluble because the soap is more polar
D) less soluble because the soap is less polar
E) more soluble because the soap is negatively charged
Correct Answer:
Verified
Q29: Vanillin is the molecule that gives vanilla
Q30: Which molecules contain a carboxylic acid?
A) CH3COOH
B)
Q31: Which compound is the MOST soluble in
Q32: Which statement about carboxylic acids is FALSE?
A)
Q33: The simplest aldehyde is formaldehyde, which is
Q35: Which compound has the highest boiling point?
Q36: At physiological pH, what is the structure
Q37: Which BEST describes the functional group(s)in this
Q38: An aldehyde, ketone, and carboxylic acid have
Q39: Which molecule is acetic acid?
A) CH3COOH
B) CH3COO-
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents