Pyrrole is a weak base because: 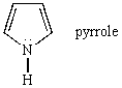
A) the nitrogen lone pair is part of the aromatic system.
B) the nitrogen lone pair is in an sp2-hybridized orbital.
C) the nitrogen lone pair is on a highly electronegative atom.
D) the nitrogen lone pair is easily deprotonated
E) the nitrogen lone pair is easily protonated.
Correct Answer:
Verified
Q1: Which of the following statements about pyridine
Q3: The structural formula for furan is:
A)
Q4: The decreasing order of basicity of the
Q5: What type of heterocycle is present in
Q6: The name of the heterocycle below is:
Q7: Which of the following molecules can be
Q8: Which of the following is not a
Q9: Which of the following statements about the
Q10: What is the structure of 2,5-dimethylpyrrole?
A)
Q11: The name of the heterocycle below is:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents