The rate-determining step in the following reaction is: 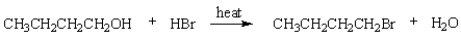
A) protonation of the alcohol
B) ionization of the alcohol to give a carbocation.
C) loss of water from the protonated alcohol to give a carbocation
D) capture of a carbocation by bromide ion.
E) displacement of water from the protonated alcohol by bromide ion.
Correct Answer:
Verified
Q22: Which of the following mixtures would NOT
Q23: The pKa of an acid whose Ka
Q24: Which reagent will accomplish the following transformation?
Q25: What is the major product of the
Q26: What type of compound is formed when
Q27: Which reagents would you use to accomplish
Q28: What is the product of the following
Q29: is the major product from the E1
Q30: Which statement is false? Tert-Butyl alcohol reacts
A)
Q32: The rate-determining step in the following reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents