Consider this chair conformation: 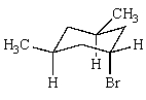 When the ring flips,
When the ring flips,
A) the bromine becomes axial and the methyls become equatorial.
B) all three substituents become equatorial.
C) the bromine becomes equatorial and the methyls become axial.
D) the ring opens up.
E) one methyl becomes axial, one becomes equatorial, and the bromine becomes equatorial.
Correct Answer:
Verified
Q30: How many monobromo products can be obtained
Q31: The least stable conformation of butane is
Q32: The most stable conformation of propane is:
A)
Q33: The compounds represented by the structures below
Q34: The least stable conformation of propane is:
A)
Q36: In the chlorination of methane, the propagation
Q37: The bond angle of a normal, tetrahedral,
Q38: Consider this chair conformation: Q39: The compounds represented by the structures below Q40: Which cycloalkane has the highest boiling point?![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents