The possible values for the magnetic quantum number ms of an electron in an atom:
A) depend on n
B) depend on 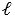
C) depend on both n and 11ef01a9_cc52_48f6_bc85_8d3f517cd313_TB6585_11
D) depend on whether or not there is an external magnetic field present
E) are 11ef0192_1f15_a623_bc85_e5393a8c0d0a_TB6585_11 1/2
Correct Answer:
Verified
Q1: The quantity Lz is related to the
Q3: The electron states in an atom which
Q4: The electron states in an atom which
Q5: The number of possible values of the
Q6: Possible values of the principal quantum
Q7: An electron in an atom is in
Q8: The magnetic quantum number mℓ is most
Q9: The number of states in a subshell
Q10: An atom is in a state with
Q11: Space quantization means that:
A)space is quantized
B)Lz can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents