The magnitude of the orbital magnetic dipole moment of an atom is ( B is the Bohr magneton, and ℓ is a positive integer) :
A) " B"
B) " B ℓ"
C) "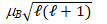 "
"
D) " B (2ℓ+1) "
E) " B ℓ2"
Correct Answer:
Verified
Q2: The possible values for the magnetic quantum
Q9: An electron in an atom is
Q10: Space quantization means that:
A)space is quantized
B)Lz can
Q10: An atom is in a state with
Q11: An electron is in a quantum state
Q12: The Einstein-de Haas experiment showed that:
A)atoms emit
Q15: The number of values of the orbital
Q17: The total number of electron states with
Q19: The number of states in a shell
Q31: In the relation µz=-m
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents