The diagram shows the energy levels for an electron in a certain atom.Of the transitions shown, which represents the emission of a photon with the most energy? 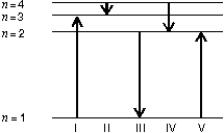
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q22: Consider the following: Q23: The figure shows the energy levels for Q25: The wave function for an electron in Q27: Take the potential energy of a hydrogen Q31: A particle in a certain finite potential Q32: A particle is trapped in a finite Q35: Take the potential energy of a hydrogen Q39: The Balmer series of hydrogen is important Q42: The radial probability density for the electron Q44: Which of the following sets of quantum![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents