An ideal gas is to taken reversibly from state i, at temperature T1, to other states labeled I, II, III, IV and V on the p-V diagram below.All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest. 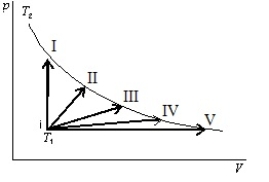
A) I, II, III, IV, V
B) V, IV, III, II, I
C) I, then II, III, IV, and V tied
D) I, II, III, and IV, tied, then V
E) I and V tied, then II, III, IV
Correct Answer:
Verified
Q1: The change in entropy is zero for:
A)reversible
Q2: A slow (quasi-static)process is NOT reversible if:
A)the
Q4: In a reversible process the system:
A)is always
Q7: Rank from smallest to largest, the changes
Q10: A hot object and a cold
Q11: The temperature of n moles of a
Q16: For all irreversible processes involving a system
Q17: The difference in entropy
Q18: Consider the following processes: The temperatures of
Q19: Consider all possible isothermal contractions of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents