The difference in energy between the n = 2 and n =1 electronic energy levels in the hydrogen atom is 1.6 *10-18 J.If an electron moves from the n = 1 level to the n=2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown in the figure below to answer this question. 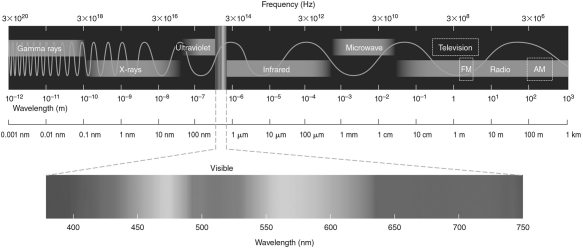
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Why do we see black lines in
Q64: Which of these planets would be expected
Q65: If a star has a peak wavelength
Q66: A spaceship approaches Earth at 0.9 times
Q67: What different processes produce the patterns of
Q69: Star C and star D have the
Q70: If the Sun's luminosity were twice its
Q71: Compare the differences between a photon of
Q72: At what peak wavelength does your body
Q73: The first five energy levels of hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents