Use the letters from the accompanying figure to answer the following questions.
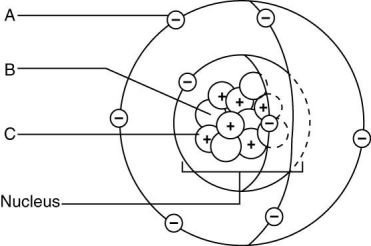
-In order for this atom to develop a positive charge, it would have to lose ________.
Correct Answer:
Verified
Q82: The acidity or alkalinity of a solution
Q83: Water can _ heat to help prevent
Q84: Use the letters from the accompanying figure
Q85: The pure form of matter that cannot
Q86: Match the following.
-a strand of 3 to
Q88: Use the letters from the accompanying figure
Q89: Match the following.
-a triglyceride that has double
Q90: Molecules that give up or donate hydrogen
Q91: Use the letters from the accompanying figure
Q92: Which solution has more free hydrogen ions:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents