The stability of 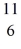 C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 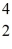 He: 4.002603 u
He: 4.002603 u 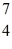 Be: 7.016928 u
Be: 7.016928 u 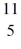 B: 11.009305 u
B: 11.009305 u 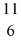 C: 11.011433 u
C: 11.011433 u 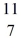 N: 11.026742 u The
N: 11.026742 u The 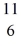 C nuclide is
C nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q23: A certain nucleus containing 8 protons and
Q28: The neutral deuterium atom, Q29: What is the binding energy per nucleon Q33: The set of nuclear reactions that power Q34: The following masses are known: Q35: If a nucleus had a diameter of Q35: What would be the expected radius of Q36: Radium-226 decays into radon-222 plus an alpha Q37: Uranium-238 decays into thorium-234 plus an alpha Q39: The carbon in your body was formed![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents